GxP AI Implementations at AstraZeneca | LinkedIn Live
Join unscripted LinkedIn Live on real-world GxP AI implementations at AstraZeneca, covering validation, governance, and regulatory perspectives. Register now.
share this

1.0. Learning from Global, Real-World GxP AI Experience
The best insights into AI and GxP come not just from frameworks or guidance but from organizations navigating this reality at scale.
Global pharma companies are:
- Deploying AI across manufacturing and quality operations.
- Engaging regulators across regions with different expectations.
- Rethinking validation and governance models.
- Balancing platform-level controls with application-level flexibility.
These experiences reveal what works, what causes friction, and where traditional assumptions fail.

2.0. A Practitioner-Led Conversation Grounded in Execution
To explore these questions through real operational experience, xLM Continuous Intelligence is hosting a live, unscripted LinkedIn conversation with:
Mr. Bob Buhlmann, Head of Quality, Digital and Computer Strategy – AstraZeneca.
Bob brings decades of experience in regulated environments, from consulting to senior leadership at Amgen, now leading quality, digital, and computer strategy at AstraZeneca.
His work covers:
- AI deployment across IT and manufacturing.
- Digital transformation and data analytics.
- GMP computer system validation.
- Data integrity and inspection readiness.
He has engaged directly with global health authorities including FDA, EMA, MHRA, MPA, and NMPA, making his perspective relevant for organizations operating across regions.
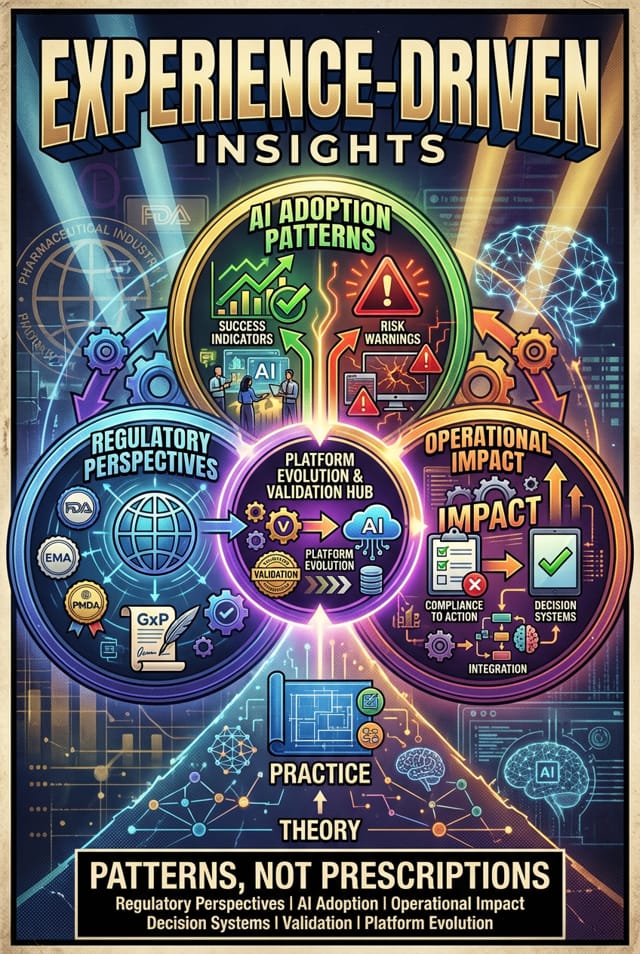
3.0. What the Live Discussion Will Explore
Instead of presentations or slides, the conversation will focus on practical, experience-driven topics like:
- How global regulators view AI in GxP-regulated manufacturing.
- Where AI adoption succeeds and where it creates unintended risks.
- How to move from theoretical compliance to operational impact.
- What changes when AI becomes part of decision-influencing systems.
- Managing validation in platform-based and evolving AI environments.
The goal is not to prescribe a single model, but to surface patterns and lessons others can adapt.
4.0. Why This Format Matters
The discussion will be moderated by Nagesh Nama, CEO of xLM Continuous Intelligence, focusing on applying AI and ML in life sciences with continuous, regulator-aware assurance.
The format targets:
- Quality and compliance leaders.
- Manufacturing and operations teams.
- IT, digital, and data leaders.
- Validation, CSV/CSA, and data integrity professionals.
This is a practitioner-level conversation grounded in execution, not theory.

5.0. Join the Live LinkedIn Conversation
If AI is on your roadmap or influencing your operations, this live discussion offers a chance to hear how these challenges are navigated inside a global pharma organization.
LinkedIn Live: GxP in the Age of AI – Real-World Perspectives
📅 Live on LinkedIn : January 29, 2026 12:00 Noon EST

share this

