xLM Process Intelligence Audit: AI-Driven GxP Compliance ROI
Discover how xLM’s Process Intelligence Audit delivers AI-driven GxP compliance, maximizes ROI, and transforms pharma workflows into success stories.
share this

1.0. Introduction
Pharma and life sciences leaders face a dual challenge: maintaining regulatory compliance and driving operational efficiency. Manual, legacy workflows slow innovation and increase the risk of human error, higher compliance costs, and missed opportunities for digital transformation.
At xLM, we believe the path to successful AI adoption in pharma starts with deep process intelligence. Our xLM Process Intelligence Audit goes beyond a standard AI readiness check; it’s a data-driven, compliance-aware, ROI-focused framework that transforms operational insights into a prioritized automation roadmap.
2.0. How the xLM Process Intelligence Audit Works
2.1. AI-Led Process Discovery (Step 1)
We deploy our intelligent, voice-enabled agent to engage those who know your processes best. Our transformation journey starts with an intelligent agent that interacts directly with selected stakeholders, ensuring we capture all relevant perspectives.
Unlike static questionnaires, our agent employs adaptive questioning logic—listening to responses, following up with targeted questions, and requesting supporting documents as needed. This dynamic interaction enables us to capture each process in detail, including key decision points, interdependencies, and execution patterns, creating a comprehensive map of your current operations.

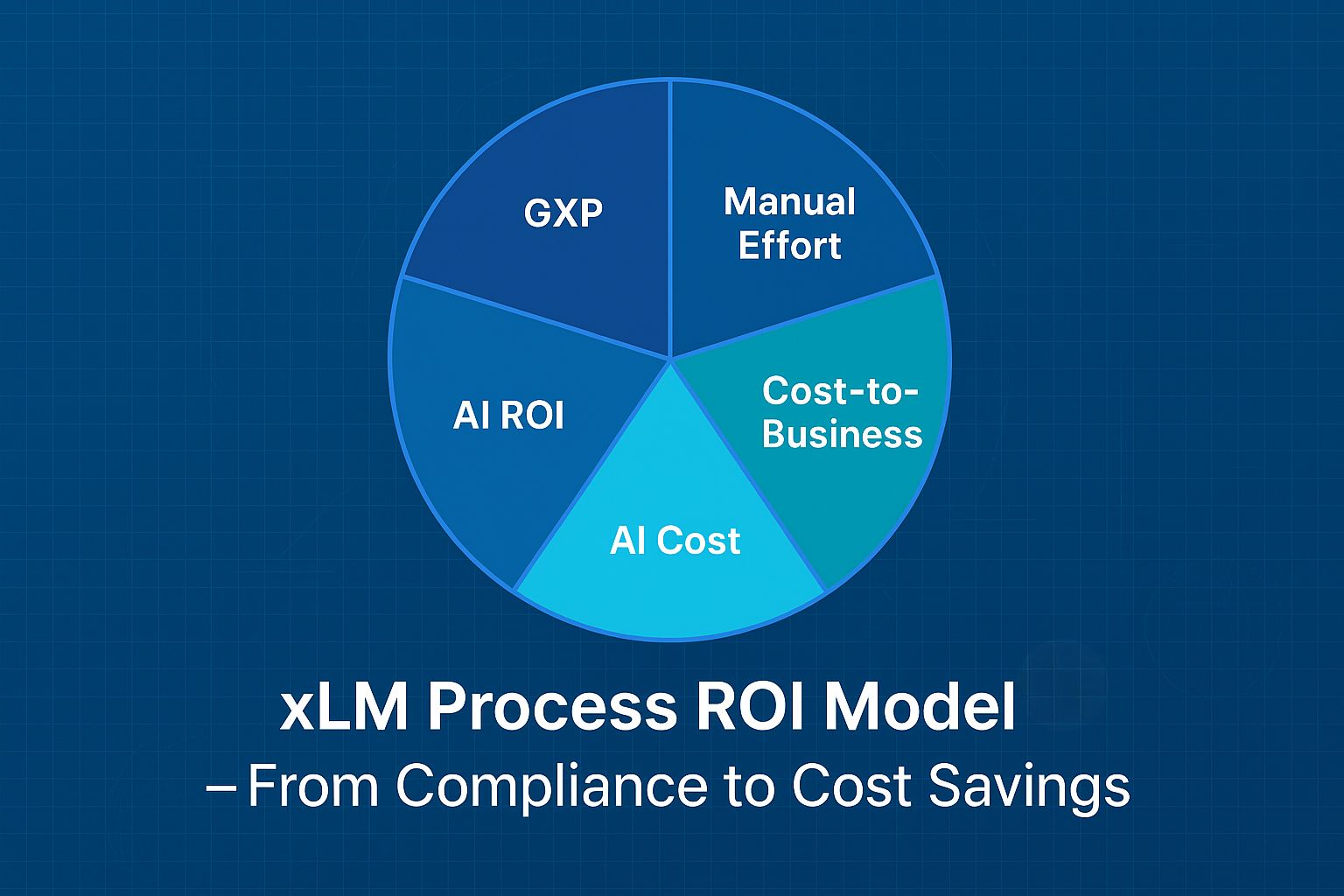
2.2. Proprietary Process Scoring (Step 2)
Our xLM Process ROI Model evaluates each process step using five metrics:
- GXP Criticality Score – Assesses the impact on Good Manufacturing, Clinical, or Laboratory Practices.
- Manual Effort Score – Ranges from fully manual to fully automated.
- Cost-to-Business Score – Combines compliance risk and operational inefficiency.
- AI Cost Score – Estimates the investment needed for automation.
- AI ROI Score – Projects financial gains over 12–36 months.
Why this matters: This model identifies and quantifies automation opportunities, enabling leadership to focus their efforts effectively.
2.3. Expert Analysis & Opportunity Prioritization (Step 3)
After the AI maps and scores, our domain experts validate each finding against industry benchmarks and regulatory requirements to ensure accuracy and relevance. We prioritize processes based on ROI potential and compliance importance, addressing the most strategic areas first. The result is a clear, boardroom-ready roadmap that outlines where and how to implement automation for maximum benefit.

2.4. AI-Driven Process Forecasting (Step 4)
With the roadmap in hand, we simulate your organization’s transition to a fully AI-enabled future. Our forecasting provides detailed compliance and business efficiency ratings before and after automation, along with savings projections for 12 and 36 months. We also calculate cumulative ROI and the expected payback period, offering a clear picture of the value AI adoption will generate. These insights are tailored for board-level decision-making, presenting a balanced view of operational efficiencies and compliance gains.
2.5. Solution Implementation with ContinuousOS (Step 5)
Once we identify opportunities and forecast their ROI, xLM goes beyond recommendations—we deliver solutions. Our ContinuousOS framework implements automation and intelligence layers to resolve legacy system bottlenecks, ensuring GxP-compliant execution across manufacturing, quality, and regulatory processes.
3.0. Additional Features for Successful AI Adoption
In addition to our proprietary scoring model, we’ve incorporated effective enterprise AI adoption methods:
- Agent readiness audit – Evaluate cultural, technical, and operational readiness for AI.
- Agent deployment support – Offer structured guidance to deploy AI for maximum impact.
- Team AI collaboration enablement – Equip teams with AI literacy, knowledge-sharing tools, and ongoing support for adoption.
4.0. Why Choose xLM Process Intelligence Audit?
At xLM, we combine industry expertise with advanced AI capabilities to deliver transformation that’s both fast and compliant. Our services are purpose-built for the life sciences sectors, ensuring every recommendation aligns with stringent GxP requirements. Leveraging our exclusive xLM Process ROI Model, we turn raw process data into quantified, board-ready insights—measuring ROI potential and compliance risk with precision. By pairing AI’s speed in data gathering with our experts’ deep regulatory and strategic knowledge, we deliver actionable roadmaps in weeks, not months, accelerating your journey to measurable business value.
- Built for pharma and life sciences – Designed for GxP compliance.
- Quantified decision-making – Rely on data for ROI and compliance risk.
- Proprietary IP – The xLM Process ROI Model™ is exclusive to our consulting practice.
- Expert and AI hybrid approach – AI gathers data quickly; experts provide regulatory and strategic insights.
- Faster time to value – From interviews to actionable roadmaps in days/weeks, not months.
5.0. Related Posts
share this

